Chromatin proteins: move or modify
Chromatin Remodelling Group
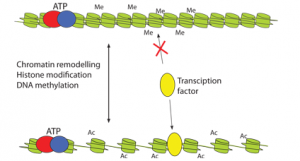
The organisation of DNA into chromosomes is important not only for packaging but also for the regulation of processes such as replication, transcription and DNA repair. Transcription factors in these processes access the DNA via enzymes that can open up the structure, either by moving chromatin proteins or by modifying them.
Cell growth and cell division rely heavily on the synthesis of proteins and require the formation of a vast amount of ribosomes, the protein synthesis machines. Ribosomal biogenesis is a general mechanism that is active to a high degree in all cells. In eukaryotic cells, the transcription of the ribosomal RNAs constitutes more than 50% of all transcription that occurs in cells. Cells have several copies of the ribosomal RNA genes, but many are silent, in particular in differentiated cells in which only 30-50% of the genes are used. Even so, these genes are subject to regulation in regard to nutrient supply such as glucose. The regulation is thought to occur on specific transcription factors involved in ribosomal gene transcription, and these are being phosphorylated and acetylated upon activation.
We have found a chromatin remodelling complex, which we have named B-WICH, which is involved in the activation of ribosomal genes transcription by remodelling sites locally and allowing other regulatory factors to bind. This leads to an acetylation at the promoter, a process that is necessary for transcriptional activation. This provides a further layer in the regulation of ribosomal genes. The B-WICH complex is an extension of the WICH complex, which is composed of the ATPase SNF2h, the WSTF (Williams syndrome transcription factor) and is involved in replication and DNA repair. The B-WICH complex also contains nuclear myosin 1 (NM1) and many RNA binding proteins. NM1 is most probably the factor recruiting some acetylating enzymes to the promoter of ribosomal genes.
One of the components in the B-WICH complex is WSTF, a protein whose gene is deleted on chromosome 7 in Williams syndrome. What haploinsufficiency of WSTF leads to is still unknown, but the few patients who have a shorter deletion, not including the WSTF gene, do not exhibit the clear craniofacial traits exemplified by those with the WSTF gene included. These findings imply that WSTF is involved in embryo development, in particular in neuronal or non-neuronal cells in brain development. Neuronal development involves many stages where key regulators, which are specific transcription factors, require specific chromatin states. Many chromatin remodelling complexes and histone modifiers are involved in neuronal development, such as the transcription-related SWI/SNF complexes where a neuron-specific version has been isolated.
During development, the epigenetic landscape changes: the chromatin condensation, the chromatin modifications and the DNA methylation patterns. Chromatin remodelling complexes are required at specific steps to pre-set the chromatin such that cell type specific genes can be induced and for signalling pathways. Ribosomal biogenesis, however, is a general process occurring in all cells. Neuronal and haematopoietic cells seem to be more sensitive than others to disturbances in many processes, including ribosomal biogenesis. At which stage of development WSTF and the B-WICH complex are involved is one of the questions my group is working on.
The SWI/SNF complexes are not only involved in transcription but also in splicing. We are investigating genes that are dependent on SWI/SNF to produce isoforms of proteins, and the consequences in different diseases, such as cancer. Infections can also trigger responses in cells, in particular immune responses. We are investigating whether chromatin remodelling or histone modifications are involved in the response to pathogens such as malaria.
Professor Ann-Kristin Ostlund Farrants
Group Leader
Chromatin Remodelling Group
Department of Molecular Biosciences
tel: +46 816 4097
www.su.se
