The many faces of (nano)particles
Adolphe Merkle Institute
For many decades scientists have prepared particulate materials for a variety of applications, such as pharmaceutical products, ceramics, paints, inks and fillers for composite materials.
Not surprisingly, most of these particles are spherical. In fact, the formation of a particle is accompanied by an energy cost associated with its surface because all atoms or molecules at the interface between two phases find themselves in an unfavourable energetic condition. The system therefore has the tendency to minimise its surface, and the sphere is the solid with the minimum surface per unit volume. Traditionally, the first step towards the preparation of particles made of two materials is to coat a simple homogeneous particle with a layer of a different material, leading to a core-shell particle.
A paradigm change in the way we conceive of particles was suggested by Pierre-Gilles de Gennes during his Nobel Prize lecture in 1991.1 De Gennes spoke about Janus grains, i.e. particles with two different faces, each bearing a different functionality. It was a great evolution from the core-shell mode because de Gennes talked about particles having two different properties present at the same time on the particle, but on well-separated regions. The first idea was to make particles half hydrophilic and half hydrophobic, somehow a particulate equivalent of an amphiphilic molecule (such as a soap molecule). It took almost a decade to develop methods for the preparation of such particles. Their properties are very different from those of their molecular counterparts. Particles interact with each other much more strongly than molecules, and similarly they almost irreversibly get pinned at the interface between two immiscible fluids. For these reasons, ultrastable particle-stabilised oil-in-water emulsions, so-called ‘Pickering emulsions’, are a highly investigated research topic, and amphiphilic particles have emerged as one of the most attractive candidates for this application.
Preparing such strange particles has, however, required a considerable ingenuity. Attaching something only on one half of the surface of a micrometre (of a few nanometres) sized particle is not at all an easy task. Chemists and material scientists have developed a variety of strategies which can be grouped into three categories.2 A first idea consists of protecting (or masking) a portion of a particle surface so as to leave only the rest of the surface available for further reaction. An astonishing multitude of solutions have been developed to mask a portion of particle surfaces, such as depositing them on flat rigid surfaces, on larger particles, on soft surfaces, or pinning them at the interface between two liquids. A second approach exploits the ability of certain macromolecules, primarily mixtures of block copolymers, to spontaneously self-organise into asymmetric objects. A third idea takes advantage of the tendency shown by different materials to phase separate, i.e. to form well-distinct domains when forced to stay together. This leads to objects that not only have two faces but that are made of distinct substances.
This last approach has widely extended the original idea of an amphiphilic particle. Material scientists have developed strategies to prepare hybrid particles made of a combination of two materials. Most of the research in the area targets the formation of heterodimers, i.e. particles made of two spherical subunits, each of a different material, permanently connected. Examples include polymer-silica, polymer-iron oxide, gold-iron oxide, etc. The choice between these different materials is often made to create particles with a combination of the properties bestowed on them by both constituents. For example, the combination of gold and iron oxide is one of the most targeted because the resulting particles will possess the extraordinary optical properties of nanosized gold and the superparamagnetic properties of iron oxides. Both materials are actively used in biomedical research, the former for optical sensing and also for phototherapy, and the latter as a contrast agent in magnetic resonance imaging and for hyperthermia. Even the combination of a polymer and an inorganic material at the nanoscale is per se fascinating, because both materials have completely different physical properties.
It is important to stress how the idea of de Gennes has so drastically changed how scientists think about particles, and especially nanoparticles. In the past, physicists have made abundant use of an analogy between atoms and spherical particles. Theories developed to explain the phase transitions of monoatomic fluids have been applied to model the behaviour of spherical particles, which offer the advantage of being much larger and slower than atoms, and therefore easier to monitor experimentally. Furthermore, the range of interactions between two particles (especially charged particles) can be varied – something impossible to achieve with atoms and a dream for physicists who aim at understanding the effect of potential on the phase transitions of spherical objects. De Gennes’ ideas have for the first time led scientists into thinking that particles might not only be the large scale equivalent of atoms but also the large scale equivalent of simple molecules.
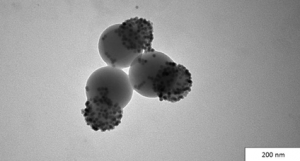
A progressive growing literature is dedicated to the preparation of colloidal molecules and patchy particles.3 The aim in this case is to build particles with complex morphology – controlled shape and a controlled amount of patches on their surface – so as to mimic the ability of molecules to form directional bonds. Molecules, proteins, and other biological molecules owe their ability to build complex structures to the formation of controlled bonds: something that particles still cannot do. Particles with many faces hold considerable promise as building blocks for the preparation of functional materials. Computer simulations are showing us that fantastic structures can be achieved if particles with well-controlled patches can be prepared. Needless to say, preparing particles in silico is much easier than in real life.
We are moving from particles with one face to those with two faces, and now towards many faces. The key is controlling where they are and how they can interact – a big challenge to face.
References
- SOFT MATTER’, Perre-Gilles De Gennes, Nobel Lectures, December 9, 1991 (www.nobelprize.org/nobel_prizes/physics/laureates)
- Synthesis and Properties of Janus Nanoparticles’, Lattuada M and Hatton T A, NanoToday 6, 286-308 (2011)
- Fabrication, Assembly, and Application of Patchy Particles’, Pawar A B and Kretzschmar I, Macromolecular Rapid Communications 31, 150-168 (2010)
Prof Dr Marco Lattuada
Adolphe Merkle Institute
University of Fribourg
Chemin des Verdiers 4, Office B224
CH-1700 Fribourg, Switzerland
[email protected]
tel: +41 (0)26 300 9525
http://www.am-institute.ch/about/people/staff/marco-lattuada
